

Interview with Jennifer Ross
Jennifer Ross is an award-winning biophysicist, principal investigator at Ross Laboratory at University of Massachusetts Amherst, and co-director of the new Massachusetts Center for Autonomous Materials (MassCAM). As an educator, she focuses on hands-on teaching techniques and advocates for women and under-represented students in STEM.
IDEX Health & Science: At the Ross Lab, you study the microtubule cytoskeleton and its effect on cellular processes. Can you tell me more about that?
Jennifer Ross: We have two lines of research, one being the biophysics of how microtubule regulators work. We’re studying a really interesting family of those regulators called the microtubule severing enzymes. You can think of microtubules as the structural lumber that makes up the cell, and you need to be able to cut them. These severing enzymes are little proteins that use ATP, and they have the ability to cut microtubules in their middle.
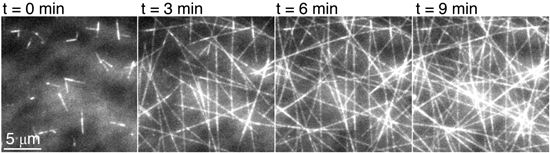
On the flip side we’re interested in how the cell self-organizes the architecture of the microtubules. We’ve been doing a number of studies looking at the self-arrangement or rearrangement using motor proteins such as kinesin-1 motors. These motors in the cell are used for cargo transport; once you’ve made an organization of microtubules, they’re not only the lumber but the highway system. You can use that highway system to take things from point A to point B throughout the cell. But what we do is put the motor proteins on a surface, and now instead of the cargo moving, the microtubule filaments themselves will glide around and we can look at what patterns they make in the presence of other interacting proteins and try to understand what organization can be made.
IDEX Health & Science: What drew you to biophysics and to this type of research in particular?
Jennifer Ross: I think microtubules are a really a good place for somebody who’s a physicist intersecting with biology because they’re structural and they’re mechanical, which is something that physicists can understand and appreciate. They are not equilibrium; both the polymers use energy to cause their destruction and the motor proteins use energy in order to go. This is a part of physics that isn’t well understood; we don’t understand how non-equilibrium-energy-utilizing things work collectively. So that’s part of this self-organization. It’s at a really nice intersection between biology and what’s called active matter in physics. We’re trying to do our part of exploring some corner of that space in physics.
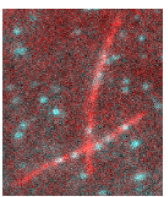
GFP-MAP65 binding to microtubules. Image courtesy of Ross Lab, University of Massachusetts Amherst. (Image from www.cell.com)
IDEX Health & Science: You use super-resolution fluorescence microscopy techniques in your research. Can you talk more about these techniques and the technologies that you are developing?
Jennifer Ross: For a lot of the biophysical studies that we’ve done, we’ve used single molecule resolution techniques in order to elucidate what the individual molecules are doing. Even if we’re doing something that’s collective motion, we’ll sometimes label a sparse few as tracers within a body that’s being organized so that we can look at how individual subunits are moving around within the organization.
In our lab we actually have multiple home-built total internal reflection fluorescence (TIRF) microscopes. We use a process called objective TIRF, where we use a high numerical aperture objective in order to create a laser beam that will come at a very steep angle. It’s a steep enough angle so that when it goes from the glass to the water of our sample, it totally internally reflects. That allows us to have quite a large number of subunits but only visualize the ones that are right at the surface. It is a great technique to allow us to see what these tracer molecules are doing within the whole system.
We also do quantitative analysis of what we’re imaging so that we can find out the numbers of molecules, how fast they’re moving, a lot of kinetics information. We can really do a lot of high-end biochemistry in the microscope with very small volumes.

IDEX Health & Science: How have Semrock products helped you in your work?
Jennifer Ross: We have a number of filters from Semrock that are pretty unique because they allow us to do multi-color imaging. It’s a multi-bandpass filter set where you allow multiple different colors in. The dichroics are really well designed so that they are able to reflect the wavelengths that we need from our laser line for our total internal reflection, and then allow through the fluorescence without a lot of bleed-through from the other channels. It makes it so that we’re not having to switch between filter cubes all the time when we’re doing multi-color imaging. For a lot of the TIRF we do, we use blue laser light and green laser light simultaneously, and we’re able to really see sequential imaging of what all of our tracer particles are doing.
IDEX Health & Science: How has the field of biophysics and microscopy advanced as a whole since you started your lab?
Jennifer Ross: When I started in grad school, I had a microscope, we were using mostly fluorescence, and I didn’t have anything automated. It was novel to have a frame grabber on our camera. As a post-doc we had a home-built automated turret system. Now the whole microscope system can be automated. Adding features such as the ability to stay in focus, to have an automatic change of filter sets—all of these things have really made it so that you can take terabytes and terabytes of data pretty easily.
A lot of technology advances have also come out in the filter world, so that we’re able to get more of the photons we want while discarding more of the photons we don’t want. The principles of optics haven’t changed. We’re mostly just automating and making things a lot easier to do.
IDEX Health & Science: You teach some advanced level short courses such as Analytical & Quantitative Light Microscopy (AQLM) at Marine Biological Labs. Would you like to talk about these courses?
Jennifer Ross: The courses for microscopy that I teach, Analytical and Quantitative Light Microscopy and the Bangalore Microscopy Course, are ways to educate a wide variety of people in the skills, techniques, and knowledge base of physics. For my course in geometric optics, I teach a half to full day of basic optics, but I don’t do traditional lectures. Instead, the students are given an optical breadboard, rails, and mounted lenses to design and build their own working light microscope. The students are mostly life science students, and giving them the opportunity to build a microscope allows them to understand more deeply how their systems work.
IDEX Health & Science: What would you consider your most significant achievement?
Jennifer Ross: I think I’m most proud of the students who are in my lab and who come out of my lab. I’ve had one particularly successful post-doc who is a professor at the University of Wisconsin-La Crosse. She’s also one of the very few African American women who are working in physics today. My job as an educator, and as somebody who can follow through with diversity and give people shots when they might not normally have had them—I take that very seriously. My lab has a lot of students of color, students from all over the world, and we’re drawing in even more students. That means that these students are coming here because they feel welcome and they feel like they can get something done, and that’s important to me.
IDEX Health & Science: What is the next challenge you hope to tackle?
Jennifer Ross: We’re starting a new center here at UMass Amherst called Center for Autonomous Materials (Mass CAM). We have a high strength in soft condensed matter physics, and we are interested in taking the next step with materials that have the ability to sense their environment, perform a computation to decide what they’re going to do, and follow through with that decision in order to perform a task. We’re interested in learning from biology in order to create smart materials of the future.
These kinds of materials can solve all kinds of problems. For instance, the next time there’s an oil spill, instead of having to drill down and cap off, you could just deploy a material that would seek out and seal the leak. If there are people trapped in a mine or in a collapsed building, you could deploy a material that would seek them out and then restructure itself to create an arch so that the people could escape from the rubble. I think that there are a lot of places where autonomous materials could go in the future, and we’re hoping at UMass that we’ll be on the ground floor of that.
