

Interview with Dr. Raimund J. Ober

Dr. Raimund J. Ober is the co-founder of the renowned Ward Ober Lab at Texas A&M University.
IDEX Health & Science: Tell us a bit about the Ward Ober Lab.
Dr. Raimund J. Ober: My wife, Dr. E. Sally Ward and I started to merge our research labs about 2 decades ago to help link the benefits of technological development to pressing needs of biomedical research. We recognized the value of an interdisciplinary environment in tackling problems relating particularly to biotherapeutics and cancer research. It became clear to us that addressing the requirements of these research areas could lead to significant technological developments that would help advance biomedical research overall.
IDEX Health & Science: Including contributing some impressive achievements in biomedical imaging?
Dr. Raimund J. Ober: Yes, this is an area where our interdisciplinary effort has really paid off. The Lab has been instrumental in developing optical approaches for viewing and analyzing cellular interactions at the near-molecular and molecular levels.
Conventional microscopy has been perfected over the years for imaging in a static environment. But the world of the cell, and the study of cellular interactions with antibodies, pharmaceuticals, and other substances all happens in three dimensions. Intercellular trafficking dynamics aren't conveniently constrained to a single focal plane. So conventional microscopy, with a single plane of focus, falls short of giving the researcher the visibility to cell interactions and dynamics that are needed. For the same reasons, phenomena related to single molecule dynamics in the cell can be very difficult to observe using conventional microscopy tools. We knew that in order to advance biomedical research, new microscopy solutions were needed that addressed the need for improved imaging at different focal planes.
IDEX Health & Science: Any solutions you think would be of interest to our readers?
Dr. Raimund J. Ober: You probably know the answer to that question, as you worked on this project as a graduate student here. We were early innovators in building systems that provide microscopic imaging that is not constrained to a single focal plane. It's termed Multifocal Plane Microscopy or MUM.
The color-coded schematic we show here will give your readers a quick way to visualize how this works. As you can see, the object of interest lies on at least two focal planes, offset from each other in the z direction. Using MUM techniques, a 50:50 beamsplitter allows us to view the first focal plane at detector 1. Image content from the second focal plane is reflected from the mirror surface and goes to detector 2.
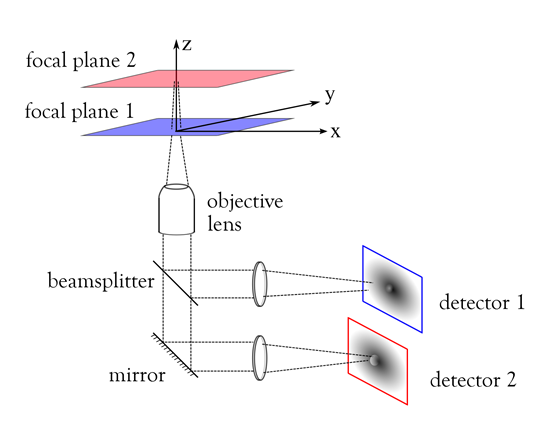
IDEX Health & Science: Allowing you to view two focal planes at the same time?
Dr. Raimund J. Ober: Yes, that's correct. I think the benefits of this method are clear to see. Without this capability to view the cell at different depths, you can easily miss activity of interest, such as a reaction to a drug or stimulus. Many intercellular events happen at different focal planes and we want to be able to view them accurately.
And, of course, with MUM you can expand the model to add more planes, allowing you to visualize up to 4 planes, or even up to as many as 8 planes without serious degradation of the image.
IDEX Health & Science: Optical filters play an important part in MUM performance?
Dr. Raimund J. Ober: Yes. Clearly we want to use filter and beamsplitter components that let us capture all of the light we can. As you know, we can't afford to waste a lot of light for these applications; many of the phenomena that we want to observe require us to acquire every photon possible.
Dr. Raimund J. Ober: We can allow quantitative tracking of single molecules in three dimensions, viewing interactions at this level in ways that just aren't possible using a single focal plane. We can identify the position of our molecule of interest with considerable accuracy.
MUM gives us capability for depth discrimination, needed in order to study cellular processes at the single molecule level. This overcomes some of the limitations of bulk studies and allows us to view single molecule interactions.
IDEX Health & Science: And MUM helps to overcome the depth discrimination problem that is a limitation in microscopy?
Dr. Raimund J. Ober: Exactly. The poor depth discrimination of the conventional microscope is a well-known limitation and can be an obstacle where super-resolution imaging is needed. Even using a high NA objective, if we consider the image of a point source in the standard microscope, it can be difficult or nearly impossible to discern the depth of that point source within a few hundred nanometers of its true focus position. That is, it can be extraordinarily difficult to determine its axial position along the z-axis. But with MUM techniques, we can acquire images of that point source at different focal planes. This additional information can help us to more clearly identify the actual depth of that point source.
IDEX Health & Science: Are there related advances in imaging that you'd like to mention?
Dr. Raimund J. Ober: We've applied our work and learning from MUM development to other areas and expanded the types of imaging tools that serve biomedical imaging. As just one example, we've developed dual-objective multifocal plane microscopy or dMUM, a technique that is particularly useful for fluorescence imaging. Basically, we can use two inverted microscopes to obtain higher photon collection efficiency over conventional technology methods.
Another important application area for MUM technology takes advantage of the capability to simultaneously image in total internal reflection fluorescence (TIRF) microscopy mode and in epifluorescence mode. In the above configuration (Figure) we replace the 50:50 beamsplitter with a wavelength-dependent beamsplitter. A protein labeled with two distinct fluorophores can be excited in TIRF mode on the plasma membrane of a cell and imaged with detector 1. The second fluorophore can be excited in epifluorescence. Thereby, we can image the protein activity deeper in the cell with the second detector. In this way we have been able to study intracellular events that precede exocytosis and, in other cases, intracellular events that follow endocytosis.
IDEX Health & Science: So your work continues to bring new capabilities to biomedical imaging?
Dr. Raimund J. Ober: Indeed. We're continuing to grow and expand into new areas. Our team of researchers is working on new ways to provide tools that support research in key areas of therapeutics and related cell research.
IDEX Health & Science: And you are involved in the upcoming Quantitative Bioimaging Conference?
Dr. Raimund J. Ober: We see this as an idea that is a long time coming. Our work to help pull this together comes from our recognition that there are many people who are working on the challenges of bioimaging in many different disciplines such as physics, biology, chemistry and engineering, but we need a forum in which to meet, to discuss, and to exchange ideas. And so, following a very successful conference in Paris in January of this year, we are continuing our efforts to bring the worldwide bioimaging community together. We're looking for productive results from the Quantitative BioImaging Conference in January, 2016 at the Delft University of Technology in Delft, Netherlands. You can learn more about the Conference at www.quantitativebioimaging.com
IDEX Health & Science: Thank you, Dr. Ober for your time and insights!
Visit the Ward Ober Lab website at www.wardoberlab.com for more detailed information on the lab and its projects.
