Constant Performance HPLC Degassing
Background
History
Many papers have discussed the role of degassing in HPLC such as a paper by Bakalyar, Bradley and Honganen from the Journal of Chromatography, 158, 1978 277–293. This paper builds on that work and its cited references to reflect on the role of in-line vacuum degassing with respect to the performance of current HPLC systems. We also present a new method by which vacuum degassing can be controlled such that the efficiency of the degasser can be set to a constant value at any flow rate, which reduces or eliminates the effect of in-line degassing on mixed mobile phase composition and reduces the discharge of solvent vapors to the laboratory atmosphere.
In HPLC separations, the reduction of dissolved air from the mobile phase is of critical importance to the stability of system flow rate, to mobile phase composition and, accordingly, to the proper identification of compounds separated by the HPLC system. For this reason, nearly all HPLC systems include some form of degassing of the mobile phase and in some cases a separate degasser channel is also used to improve accuracy of the autosampler and even the performance of seal wash systems.
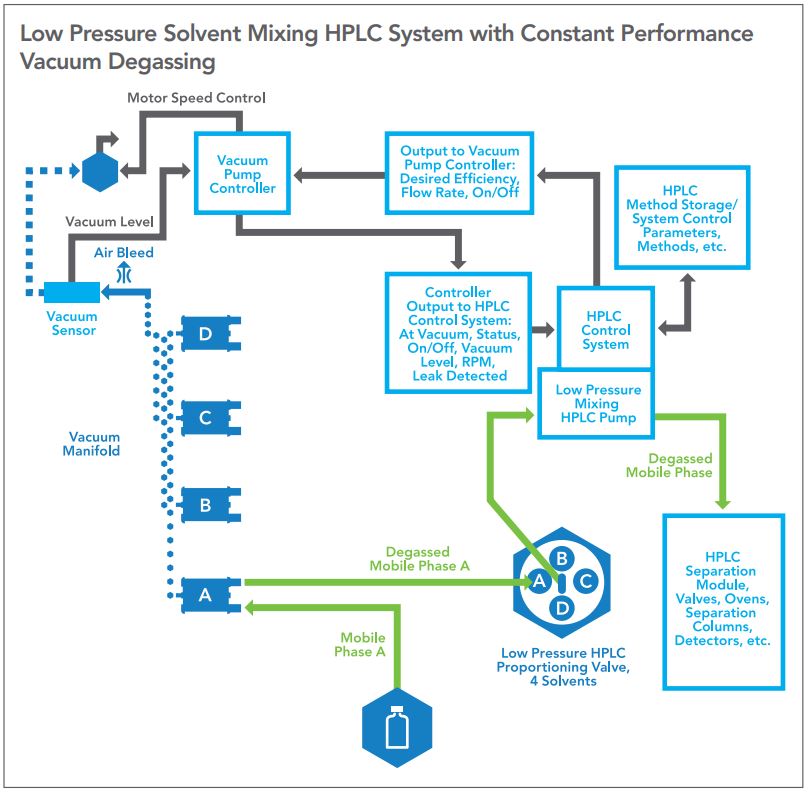
Figure 1 — A generic low pressure gradient mixing HPLC system.
As a review, most HPLC systems fall into two categories: those which mix solvents prior to entering the pump known as low pressure mixing pumps, and those which mix solvents after the pump known as high pressure mixing pumps. The general configuration of both types of systems are shown in Figures 1 and 2.
In the Low Pressure HPLC mixing system, a mixture of solvents is created by timing the opening and closing of individual solenoid valves during the intake stroke of the pump. The individual air saturated solvents then are moved to the inlet check valve of the pump through a connecting line wherein the first solvent is exposed to the second, third, or fourth solvent as sequenced by the HPLC controller. This transfer occurs at atmospheric pressure or slightly lower due to the pressure drop through the transfer line to the pump inlet. The individual solvents then must be degassed to prevent bubble formation and accompanying mixture errors.
In a High Pressure mixing HPLC system, each solvent has its own pump and no mixing of solvents occurs before each pump’s inlet check valve, instead, mixing occurs at a point after each pump and ahead of the injection valve. At the mix point, each component of the mixed mobile phase is at high pressure and outgassing of the mixed mobile phase is suppressed until it returns to atmospheric pressure. Still, high pressure mixing HPLC systems benefit from degassing the mobile phase due to the potential for cavitation and malfunction of the inlet check valve of the pump, especially in the case of gravity operated check valves.
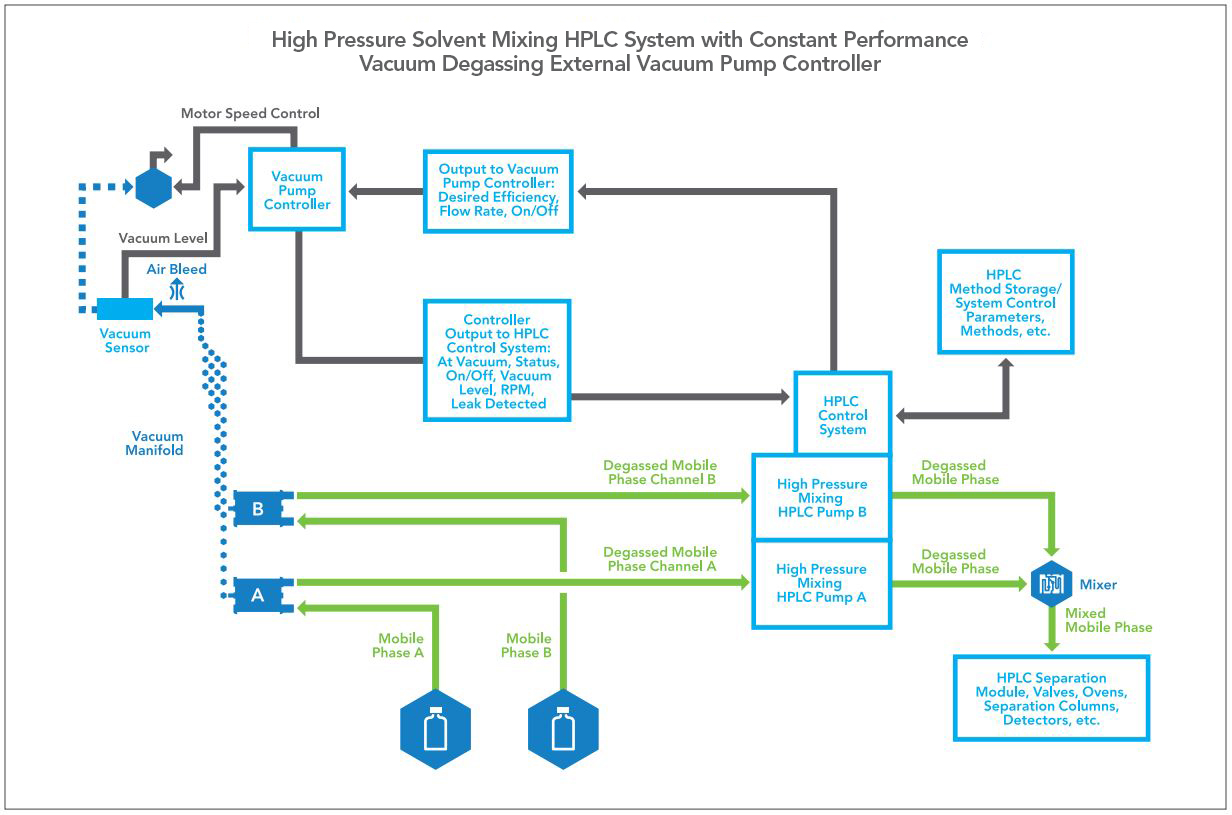
Figure 2 — A generic High Pressure mixing HPLC system showing the relationship between the degasser and the inlet of the HPLC pumping channels.
Since high pressure mixing HPLC systems utilize two pumps supplied by pre-mixed individual mobile phases, the efficiency of the degasser may not be as great as the efficiency of degassing for low pressure mixing HPLC systems. However, gravity operated inlet check valves are sensitive to cavitation bubble interference during the pump stroke, therefore all HPLC manufacturers have chosen to degas the mobile phase entering the pump to the same degree as required for low pressure mixing systems. Additionally, because the two different mobile phases are not combined until after the pumps and at high pressure, outgassing will occur when the combined mobile phase returns to atmospheric pressure after the HPLC column, either at the column outlet or as the mobile phase enters a mass spectrometer nebulizer. This additional benefit of operating the HPLC system below the point at which methanol-water mobile phases will outgas ensures the lowest possible detector noise.
The Foundations of Degassing Solvents for HPLC
In 1976 Junji Tukunaga published the Ostwald coefficients for the solubility of oxygen and for nitrogen in various alcohol-water mixtures through the range of 0% alcohol to 100% alcohol as a mole ratio. This seminal publication demonstrated the degree to which methanol plus water mixtures need to be degassed to prevent bubble formation and formed the foundation for today’s in-line degassing for HPLC solvent mixtures.
Although his work dealt with alcohol — water mixtures in general, the use of his methanol — water data has proven to be more than adequate to describe the case for all known solvent combinations in use by HPLC systems today, either when using high pressure or low pressure mixing.
Figure 3 utilizes Tokunaga’s data in which we have changed Tokunaga’s data from mole ratio to volumetric percentages as used in everyday HPLC mixing.
Tokunaga’s data set has been plotted using the total contribution of dissolved air to the mixture of methanol and water as the ratio changes from water to methanol. The difference between the upper solid red line and the Ostwald coefficient data lines represents the supersaturation of the mixtures with dissolved air.
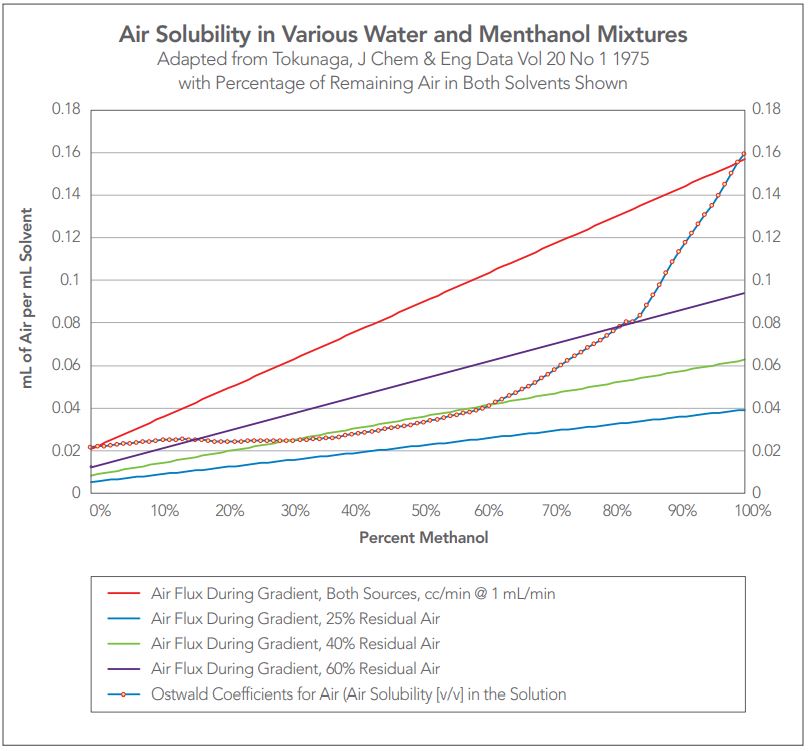
Figure 3 — Tokunaga as volume % showing the effect of degassing on the level of supersaturation of the mixtures with dissolved air.
Three example lines are also presented in which the amount of air remaining in water and in methanol is reduced by degassing. The upper purple line represents the total amount of air resulting from degassing both water and methanol to 60% residual air. The middle green line represents 40% residual air and the blue line represents 25% residual air. Clearly, 60% residual air still will produce a supersaturated solution when water and methanol are mixed between perhaps 15% and 85% methanol. When the solvents passing through a degasser contain 40% residual air, there is only slight supersaturation between perhaps 35% and 65% methanol. And at 25% residual air, no outgassing potential remains.
From Tokunaga, the actual concentration of air in the alcohol plus water mixture which will not outgas at atmospheric conditions is 38% when expressed as the maximum vacuum pressure [760 mm Hg (atmospheric pressure) X 38% = 289 mm Hg]. Generally, all HPLC degassers are designed to meet the target 38% residual air (62% efficiency of removal) at a specific flow rate and applied vacuum to meet the instrument design requirements.
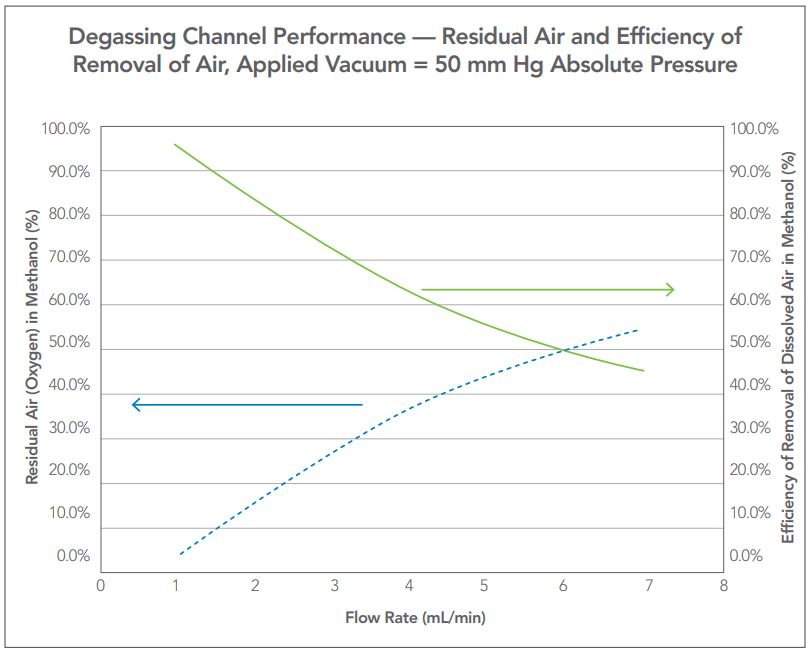
Figure 4 — Expression of performance as residual air and efficiency of removal of air using Oxygen as an indicator.
Degasser Performance
In-line degassers operating at a single vacuum level will remove air from a mobile phase more efficiently at a low flow rate and less so at a higher flow rate due to the longer residence time. The characterization curve for degassers is either expressed in terms of residual air as determined by UV absorbance of oxygen in methanol, or by using a dissolved oxygen probe and water. A typical degasser characterization curve is presented in Figure 4.
As seen in Figure 4, there are two different methods of expressing the performance of a degasser, one expressed as residual air as described by Tokunaga, the other expressing the performance as efficiency
Selectivity and Pervaporation: A Note About the Nature of In-Line Degassing
Membranes of all types (in the case of HPLC degassing, Teflon AF, or PTFE) will allow air through the membrane in accordance with Henry’s law, Dalton’s law, and more specifically to mixed mobile phases, Raoult’s law. The selectivity of a membrane is controlled by the polymer type specifically and its selectivity for one molecule over another.
Specifically for Teflon AF, the main driving force for permeation is molecular size, its solubility in the membrane, and the concentration differential (or pressure differential) driving the permeation. The combined movement of solvent molecules from the mobile phase to the vacuum side is known as pervaporation. The greater the concentration differential, the greater the driving force for pervaporation. In vacuum degassing, the level of vacuum represents the concentration of all permeating components from the mobile phase.
Influence of Pervaporation on Concentration
Increasingly, manufacturers of HPLC systems have extended the method flow rate range of HPLC systems to include micro-flow separations from microliters per minute to the common milliliter per minute range. This extended range capability has placed a new demand on the role of the degasser and brought about a new method by which the degasser can perform its role in conditioning HPLC solvents without significantly changing the concentration of a pre-mixed mobile phase.
In traditional vacuum degassing, a vacuum setpoint of 50 to 80 mm Hg absolute pressure is used to establish the working vacuum for the degassing system. When the vacuum is set to a fixed pressure below the vapor pressure of the majority of common HPLC solvents, the vacuum pump continually removes both dissolved atmosphere and solvent vapors controlled by the permeability of the membrane for each. In the case of very low flow rates, air dissolved in the mobile phase quickly passes into the vacuum side and is exhausted by the vacuum pump. So long as the vacuum remains applied, solvent vapor permeates into the vacuum space and is continually pumped away to the atmosphere as well. Since the solvent pervaporation rate is continuously refreshed by the solvent supply bottle, the vacuum pump continues to remove solvent or individual mobile phase constituents continuously so long as the vacuum is below the vapor pressure of the mobile phase. In the case of a single component mobile phase, there is no concentration effect. In a pre-mixed mobile phase, differential pervaporation will change the concentration of the mixture proportional to the residence time, volume of the degasser, and the rate of pervaporation of one component of the mixture versus the other(s).
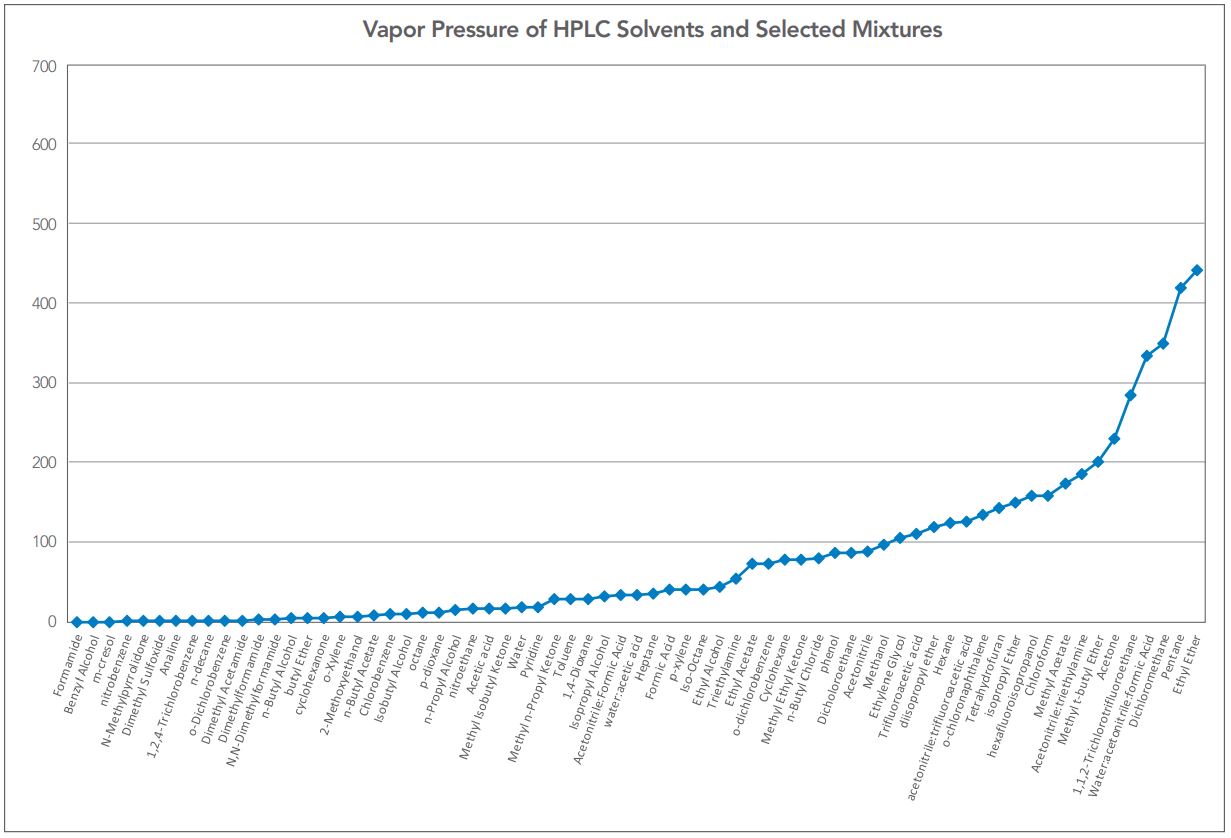
Figure 5 — Vapor pressures of most HPLC solvents.
It is therefore desirable to suppress pervaporation by controlling the vacuum side of the degasser to as high a pressure in the vacuum chamber as possible without reaching a point at which outgassing will occur in the HPLC system.
A method to control the rate of pervaporation of the mobile phase which might result in concentration changes over time for a mixed mobile phase such as acetonitrile and trifluoroacetic acid was discussed in detail in US 8017016. An example of this method adjusts the vacuum level to above the Raoult’s law calculated vapor pressure of the solvent and the acid. Raoult’s law (ideal solutions) scales the vapor pressure of each volatile component of a solution in accordance to its mole fraction in the solution.
A vapor pressure plot of most HPLC solvents is included in Figure 5, for reference.
A Solution Providing an Optimal Level of Degassing While Minimizing Mobile Phase Concentration Changes
Current Practice
Since the introduction of in-line membrane degassing in the early 1990s, each manufacturer of an HPLC system has specified a degasser’s performance to meet the target performance of the HPLC system using a single vacuum setpoint. Flow rates less than this specification will have less dissolved atmosphere and those above will have more depending on the native efficiency curve of the degasser.
However, we find that additional characterization of the degasser at various vacuums, different from that specified by the manufacturer, reveals a new range of performance which can be related to different selected performance levels.
The Case for Lower Vacuum (Higher Degassing Pressures)
All current HPLC degassing systems operate at a single fixed setpoint for the applied vacuum for all methods and flow rates. Typically this value has been set to 50 mm Hg absolute pressure, but other pressure/ vacuum levels are also used. Vacuum degassing systems set to a single high vacuum setpoint (e.g. 50 mm Hg) are designed specifically to meet the upper flow rate range of the individual HPLC system and all channels are set at one vacuum level. A single channel example degassing curve is illustrated in Figure 4. Referring to Figure 4 in view of Figure 3, one can see that the example degasser will degas a single solvent to the 60% efficiency (40% residual air) at 4 mL/min in a single channel. At flow rates lower than 4 mL/min, the amount of air contained within the solvent becomes increasingly less (efficiency becomes greater).
Since degassing is primarily to prevent bubbles when the solvents are mixed and to also prevent check valve interference, “over-degassing” the mobile phase works, but can come at the cost of pervaporation of the solvents contained in unused degassing channels and potentially changing the concentration of the active mobile phase.
The Case for a Flat Film Design
There are several advantages to using a flat film membrane instead of the common tubular membranes. Diffusion theory (Fick’s Law) states that the shorter the diffusion path, the less time it takes for a gas molecule to travel from a high concentration area to a low concentration area. Diffusion of dissolved atmosphere through the mobile phase to and then through a membrane into an applied vacuum is affected by both the diffusion path length through the fluid and the diffusion path through the membrane.
A vacuum degasser manufactured using tubing incorporates several interactive design elements affecting efficiency and flow restriction. Wall thickness (membrane diffusion path) cannot be too thin or the degassing coil will kink during manufacturing. The ID (fluid diffusion path) cannot be so small that flow restriction within the degasser becomes problematic. Over the years, this interaction between the elements of fluid flow has resulted in multiple designs, sizes, and in some cases, resulted in the need to place multiple tubes in parallel in each degassing channel. A properly executed flat film design eliminates many of the design limitations of a tubular membrane degassing channel.
The primary advantage of a flat film membrane is that it can be made much thinner than the wall of a tube which must be coiled. This makes the film proportionally more efficient. Secondly, the dissolved gas diffusion path (fluid layer thickness) can be made to be less than the diffusion path through the fluid in a practical tubular degasser. Efficiency is then a function of the surface area of a membrane, its thickness, and the thickness of the fluid path. Flow restriction is a function of the quality of the fluid distribution and the thickness of the fluid path. These advantages of a flat film combine to create a highly efficient degassing channel for both high and low pressure mixing HPLC systems (Figures 1, 2).
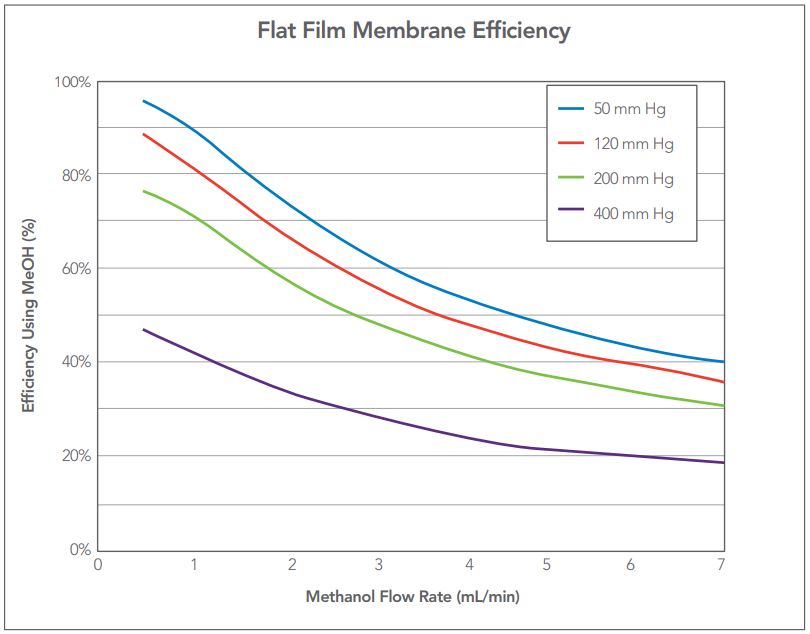
Figure 6 — Characterization curves showing efficiency vs. flow rate at 4 different pressures (vacuum levels) for a single channel flat film degasser PN 9000-2071.
Achieving a "Universal" Degassing Solution
The optimal degassing solution would achieve any level of performance for any flow rate within the range of applied vacuum. Primarily the degassing channel must have enough performance to meet the requirements of any analytical scale HPLC system. Secondarily, the degasser should minimize solvent vapors moving across the membrane (pervaporation) to reduce concentration changes in mixed mobile phases and at the same time minimize the amount of solvent vapor discharged into the laboratory atmosphere. There are two key elements which contribute to this “universal” degassing solution:
- An exceptionally high-performance degassing chamber
- A controllable vacuum pumping system
IDEX Health & Science has introduced a flat membrane degasser that utilizes a Teflon AF-based membrane1 with sufficient surface area to degas solvents to the highest degree necessary for analytical scale (less than 10 mL/min flow rate) HPLC systems. Combined with a unique flow channel design2 and significantly shorter fluid and membrane diffusion paths, the rate of degassing is enhanced over tubular designs in a small package.
A vacuum pump control algorithm is used to adjust the degasser vacuum depending on flow rate. This method of vacuum control achieves a desired efficiency for any flow rate within the range of the applied vacuum. Simply raising or lowering the pressure in the degassing channel(s) changes the degassing performance to that needed by the HPLC while simultaneously reducing pervaporative changes in the mobile phase. This method of adjustment achieves an appropriate level of degassing for any HPLC system design and separation method. To achieve this performance, the degassing chamber must be characterized.
Characterizing the Flat Film Membrane Degasser
A mathematical representation of the performance of the degassing channel versus the applied vacuum level is derived from data tying degassing performance to the flow rate of the running HPLC separation and is stored in either the degasser controller or in the associated HPLC control system.
Characterizing the Degassing Chamber
Step 1: Using the methanol-oxygen charge-transfer complex at either 210 nM or 215 nM, the efficiency of a degassing chamber type is determined at 6 flow rates and 4 applied vacuums.
Referring to Figure 6, the maximum flow rate at which the individual channel can achieve 30% residual air (70% efficiency) when operated at 50 mm Hg absolute pressure (vacuum level) is approximately 2.5 mL/min. This will sufficiently degas a gradient or any isocratic low-pressure mixed methanol-water system up to 5 mL/min (using one channel for each solvent). Since an efficiency of 62% is required to prevent outgassing, an HPLC system equipped with a degassing system utilizing this model degasser and operated at 50 mm Hg absolute pressure could be expected to use methods flow rates as high as 7 mL/min without bubbles forming after mixing (methanol plus water).
Step 2: Plot efficiency vs. vacuum level for each flow rate.
Figure 7 is a re-plot of the characterization data from Figure 6 showing the relationship between the applied vacuum and the degassing efficiency for each flow rate.
To convert the data plot in Figure 7, the formula for each curve is obtained from which the applied vacuum for a desired flow rate through a single degassing channel can be calculated from any desired efficiency of degassing. The formula for the curve of each flow rate is then determined and used to solve for vacuum vs. flow for any given degassing efficiency.
Step 3: From the Efficiency – Vacuum curves equations, solve for vacuum using the desired efficiency and flow rate. Plots of the resulting curves are shown in Figure 8.
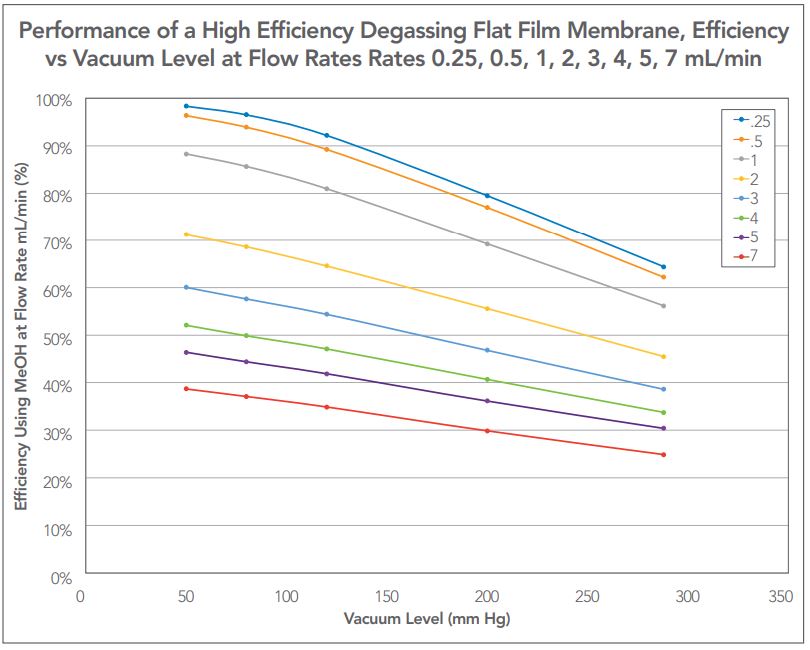
Figure 7 — Flat film membrane degassing channel efficiency versus applied vacuum at all calibrated flow rates
From the graphical analysis of the data in Figure 8 the formula for each curve at each plotted efficiency is obtained. Once derived, flow rate may be used to determine the applied vacuum for a given efficiency of the degasser. From the annotations in Figure 8, the upper limit for vacuum applied to the membrane should be limited to 288 mm Hg as pressures above that level risk outgassing methanol – water in-pump mixes when atmospheric pressure is 760 mm Hg. The formula for each curve then can be used to calculate the vacuum level for any method flow rate where the individual channel flow rate is half the method flow rate.
Step 4: The formula of each curve plotted in Figure 8 links flow rate to output vacuum level. Once a degassing chamber type is characterized, the vacuum level applied to the degasser is a function of the desired efficiency of the degasser and the flow rate of the method.
Degassing performance can then be tuned using vacuum control to cover the entire performance range of an HPLC system by adjusting the applied vacuum as a function of method flow rate. In this manner, a target efficiency can be constantly assured at any flow rate while ensuring changes in the concentration of the mobile phase are minimized. From microliters per minute to milliliters per minute, the chromatographic performance of the HPLC is assured.
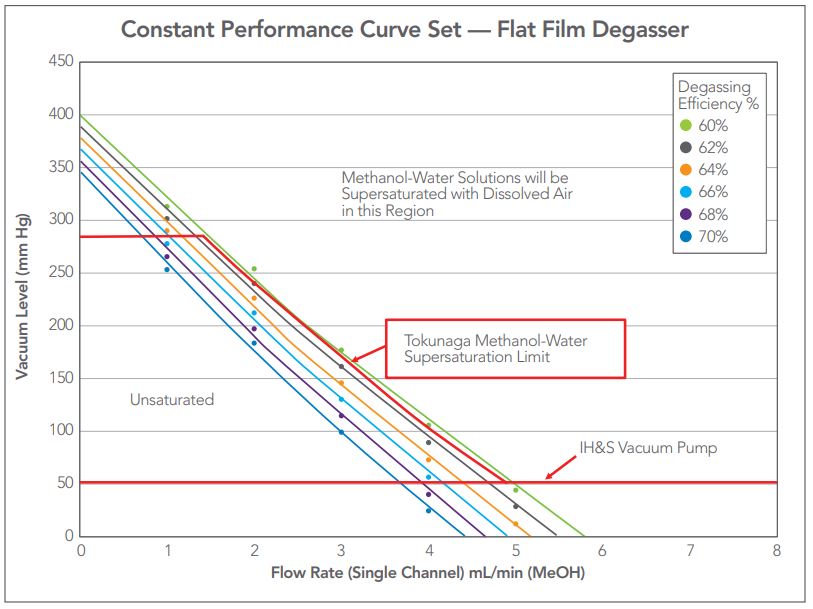
Figure 8 — Constant Performance curve set for the flat film membrane degasser. The plot shows Tokunaga’s upper limit of methanol-water mixture supersaturation at 760 mmHg and the lower limit representing the maximum performance of the IDEX Health & Science 2-stage vacuum pump.
Practical Application of the Universal Degasser
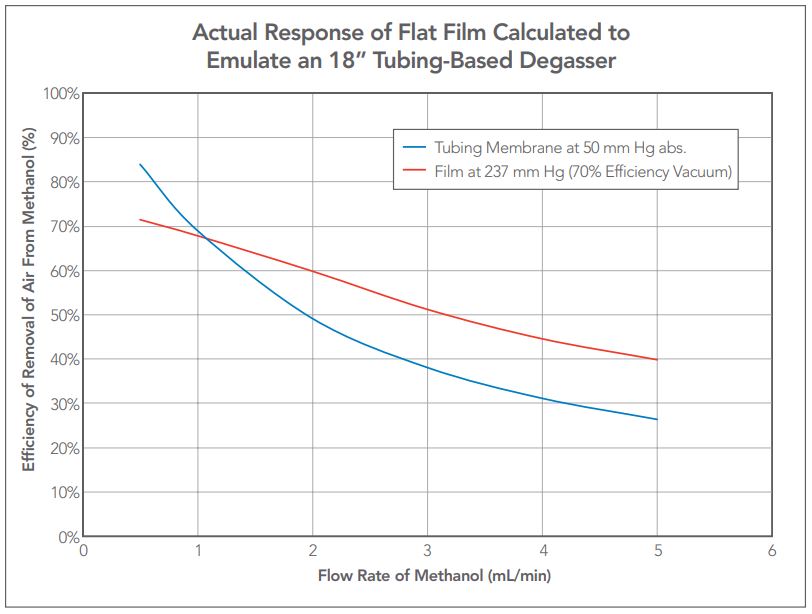
Figure 9 — Comparing a predicted vacuum level efficiency of 70% at 1 mL/min against a standard 18” degassing channel operating at 50 mm Hg vacuum. Flat film membrane efficiency specified at 70% and the calculated vacuum level at 1 mL/min was 237 mm Hg.
A demonstration of the power of the characterization of the flat film membrane degasser is shown in Figure 9 where the performance of a tubing based IDEX Health & Science vacuum degasser is compared against that of the flat film. Note the vacuum levels are dramatically different but the performance of the flat film membrane matches that of the tubular degasser at the OEM customer’s desired flow rate and efficiency of 1 mL/min with 70% efficiency (30% residual air).
This demonstrates that any degasser can be characterized, and the resulting sets of data can be used to control the vacuum degassing system from the inputs of efficiency and method flow rate.
A Novel Implementation of Vacuum Degassing Control (Patent Pending)
To put the characterization in to practice, the degasser control can be integrated with the chromatography system in three different ways:
- The HPLC sends only the method flow rate to the degasser which maintains a fixed efficiency. The on-board vacuum pump control calculates the appropriate vacuum.
- The HPLC sends the desired efficiency and method flow rate to the degasser. The on-board vacuum pump control calculates the appropriate vacuum.
- The HPLC sends only the vacuum level to the degasser: The HPLC system uses the degassing control algorithm to calculate the vacuum level from the method flow rate and desired efficiency.
In Figure 9, the degassing control algorithm contains the upper limit for vacuum where supersaturation becomes possible along with the lowest pressure the vacuum pump can achieve.
This control methodology provides the following:
- A means to select a given efficiency of degassing for any HPLC system.
- A link between the chromatographic method flow rate to establish the appropriate vacuum level.
- The same degassing efficiency for any flow rate within the range of the vacuum system.
- The highest possible pressure minimizing solvent loss to the laboratory atmosphere.
- Reduction or elimination of mobile phase concentration changes due to pervaporation.
- Reduced vacuum pump wear when operating at higher pressures in the degassing channel due to lower pump RPM.
Empowered by the Constant Performance Vacuum Control the New IDEX Health & Science Flat Film Membrane Degasser Has the Following Advantages
- Lower flow restriction than tubing based degassers.
- Non-metallic flow path, which enables universal application of a single type of degasser to multiple types of HPLC systems.
- Small form factor with no internal tubing fittings to leak.
- Lowest vacuum volume to limit initial pervaporation of volatiles.
- Universal coverage of all flow rate ranges to as great as 8 mL/min or more depending on style of HPLC system.
- Universal solvent compatibility, except for solvents known to adversely affect Teflon AF (see solvent compatibility guide).
- Solvent flush-out similar to tubing style degassers.
- Flow restriction is constant regardless of applied vacuum.
- Lower environmental impact: High pressure vacuum degassing reduces or eliminates solvent vapor discharge into the laboratory atmosphere.
References
- US 9962661, US 9381449, US6596058
- US 10143942
Authors
Carl W. Sims, David Wert, Quan Liu, Jeremy Hayes, and Saba Jazeeli
