Glossary of Terms
This glossary of terms has been compiled for your convenience. To view a term’s description, click the square next to it from the list below.
Activity
In ADSORPTION CHROMATOGRAPHY, the relative strength of the surface of the packing material. There are ways, which vary as to the nature of the packing material itself, to modify and control the activity of this surface.
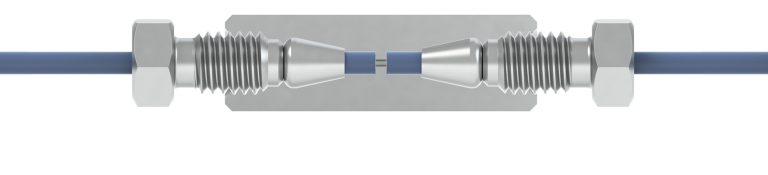
Adapter
A union with different threads on each end; generally used to connect two different types of tubing together.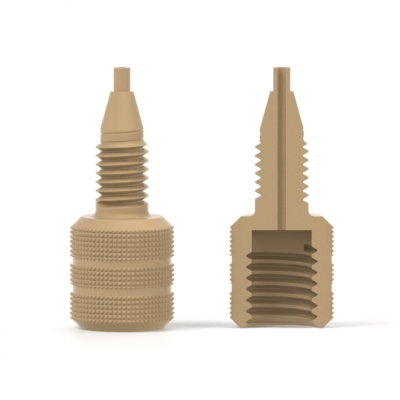
Adsorb
To hold molecules of gas or liquid to the surface of a solid material. Not to be confused with Absorb, which is the process of combining or merging one object into another (as in water).
Adsorbent
Refers to the material often used to pack columns used in ADSORPTION CHROMATOGRAPHY, most often alumina or silica gel.
Adsorption Chromatography
A chromatographic method in which the molecules are retained through the interaction of the polar functional groups of the sample components and the functional groups on the surface of the packing material.
Amino Phase
A specialized stationary phase often used for selective normal phase chromatography, in which the solutes interact with amines.
Analytical Column
Typically, the main column used in the HPLC system to separate sample components; often referred to as the “heart” of the HPLC system.
Anneal
To take the brittleness out of metal, plastic or certain carbon composites. Performed in the preparation of new products or in their restoration, annealing is accomplished via a heat treating process.
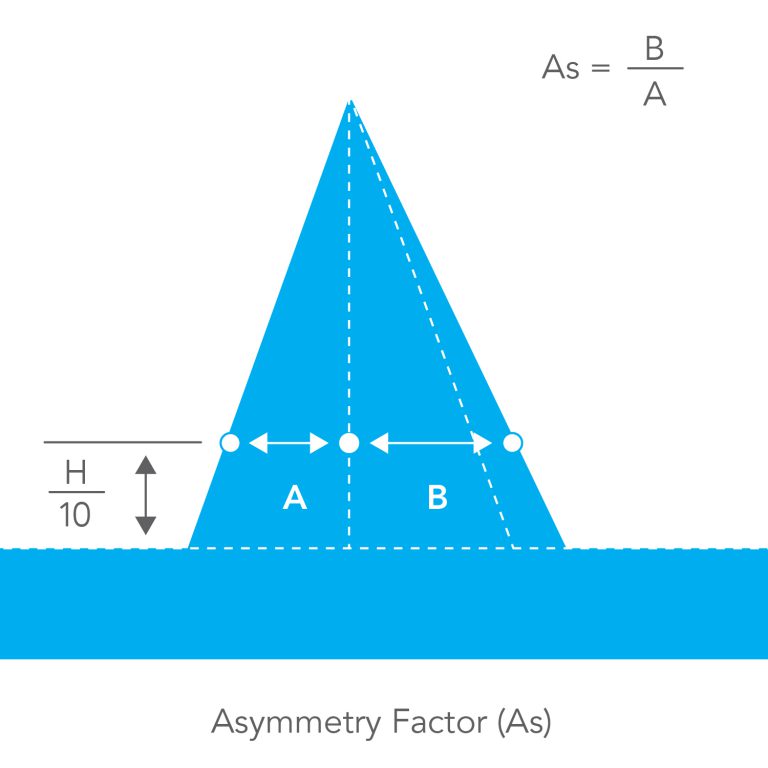
Asymmetry Factor
A factor used to describe the shape of a chromatographic peak. While theory assumes a symmetrically-shaped peak, in practice the peak is often asymmetrical. The equation used to describe this factor is determined at 10 percent of the peak height. At this level, the factor is defined as being the ratio of the distance between the peak’s apex and the back of the peak’s curve to the distance between the peak’s apex and the front of the peak’s curve.
Back Pressure
A phrase used to describe the pressure required to force fluid at a determined flow rate along a system’s flow path, typically expressed in psi, bar, atm, or MPa.
Back Pressure Regulator
A device typically used after the detector to maintain a positive pressure on the flow cell, thus minimizing solvent outgassing problems in the detector. It can also be used as a PUMP PRELOAD, to help the pump’s check valves operate more efficiently.
Band
A visual deflection along a chromatogram’s baseline, representing a sample component. Also known as a PEAK.
Bandspreading
References the dilution of the BAND as it moves from the injection valve through to the detector.
Band Width
The width of the BAND measured at its base.
Bar
A unit of pressure equal to one atmosphere; also equivalent to about 15 pounds per square inch (psi) or 0.1 Megapascal. (Note: See our Pressure Conversion Chart to make more exact cross-references between pressure units.)
Baseline
The baseline is the line drawn by the recording device representing the signal from the detector when only mobile phase is passing through. It also represents the point from which calculations are often made on peaks to determine quantitative data.
Baseline Noise
Often evidenced as variations along the chromatographic baseline, this is usually due to electrical noise or temperature fluctuations, but can also be due to outgassing in the detector’s flow cell, as well as poorly mixed mobile phase.
Binary-Solvent Mobile Phase
A MOBILE PHASE composed of two solvents being mixed together. Many mobile phases are of this type; also called Binary Mobile Phase.
Biocompatible
Refers to that special quality of some materials allowing them to come into contact with biological materials without changing the materials’ bioactivity.
Body
Refers to the main section of the fitting, into or onto which nuts and ferrules (if appropriate) attach.
Bulkhead Fitting
A fitting designed to extend through an instrument panel; includes a lock nut to hold the fitting in place.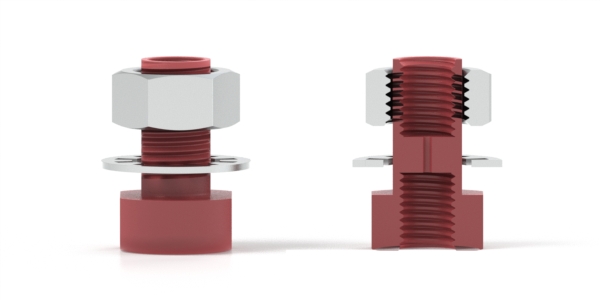
Capacity Factor (k’)
A way of expressing the retention of the sample components. It is determined by subtracting the COLUMN DEAD TIME from the retention time of the peak of interest, and dividing that by the column dead time.
Capillary Electrophoresis (CE)
A special type of chromatographic analysis which employs the use of electricity to selectively separate charged species comprising a sample. It is a powerful technique that produces sharp bands with minimal tailing, with the main difficulty being the sensitivity threshold.
Capillary Electrochromatography (CEC)
A newer type of chromatographic analysis which combines the benefits of CAPILLARY ELECTROPHORESIS and HPLC. Typically, with standard HPLC, there is a pressure limitation with the equipment which limits the number of theoretical plates which can be employed. Because flow is driven electro-osmotically in CEC analyses, there is no theoretical plate threshold.
Carbon Load
Term usually used to describe the degree to which the available silanols on the column packing’s surface have reacted and been replaced with the bonded phase; the higher the carbon load, the lower number of residual silanols.
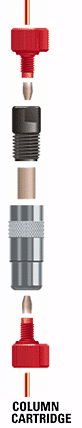
Cartridge Column
A guard or analytical column that comes pre-packed, and is placed in a reusable holder for use. Because of their design, cartridge columns are often easier to change, less expensive, and more convenient than conventional columns.
Channeling
A phenomenon occurring when voids are evidenced in the column’s packing material, often due to improper packing or mishandling of the column.
Check Valve
A device inserted into a moving liquid stream that allows flow of the stream in only one direction; often built into the HPLC pump.
Chemical Garbage
The contaminants in a sample that can foul up the LC column by irreversibly sticking to the bonded phase. A guard column is often used to filter the chemical garbage out of the flow path.
Chiral Stationary Phases
Specialized stationary phases used in chromatography to separate ENANTIOMERIC COMPOUNDS. These are generating much interest in today’s chromatographic arena.
Chromatogram
A plot stemming from a detector’s signal output, typically versus time. It is identified often by a BASELINE offset by a series of PEAKS or BANDS.
Chromatography
Literally “color movement,” a term describing a separation technique that occurs based upon the difference of interaction of sample components with two phases – a “stationary” phase (or non-moving) and a “mobile” phase (or moving).
Chromatographic Conditions
Those parameters that describe how an analysis was achieved, for the purpose of potential future duplication for verification purposes.
Column
Typically a tube of sorts that contains the STATIONARY PHASE of a chromatographic system.
Column Dead Time
The time it takes for solvent molecules or unretained sample bands (for which capacity factor =0) to pass through the column. Uracil is often used to measure the column dead time for reversed-phase column.
Column Packing
The particulate material packed inside the column; also called the STATIONARY PHASE. This usually consists of silica- or polymer-based particles, often chemically bonded with a chemical function al group. For analytical work, 3µm or 5µm spherical particles are used; for semi-preparative, 10µm or larger spherical or irregular particles are favored. The most common packings are as follows:
- Octadecyl (ODS, C18) the most popular phase, used for reversed-phase HPLC
- Octyl (C8) more polar than C18, also a popular reversed-phase column
- TMS (methyl, C1) weakly retentive in reversed-phase
- Silica (Si) unbonded silica used in normal phase mode, popular for preparative LC.
- Phenyl, Cyano, Amino specialty phases used for specific applications.
Column Performance
A column’s efficiency, often expressed through a THEORETICAL PLATE value, used to describe how well theoretically a column will resolve sample components.
Compression Columns
This column format uses a non-threaded tube that has a three piece compression end fitting. The end fitting consists of a male fitting, a female fitting and a ferrule. The way the end fitting is held on to the tube is sliding the tube into the fitting. The tubing is then tightened so that the ferrule bites on to the tube. The ferrule is all that holds the fitting from coming off the tube under pressure.
Compression Hardware
Compression hardware relies on friction between the ferrule and tubing body to maintain the high pressure seal. The word “compression” is associated with this style of hardware because the friction is created by physically compressing the ferrule into the tubing by applying torque. The hardware is made from 316 grade stainless steel for inertness and corrosion resistance.
Concentricity
In HPLC, the degree to which the thru-hole of a part has a common centerline with the part. This is critical when aligning small-bore tubing with a union’s thru-hole. If the thru-hole is not concentric, the flow path may be restricted.
Conductivity Detector
A specialized detector which measures the conductivity of fluid passing through its flow cell. Useful in a variety of applications, these detectors can often provide analytical data on sample components that other, more standard detectors, cannot provide.
Cracking Pressure
Typically relates to a check valve or back pressure regulator; describes the minimum amount of (positive) pressure required to open and maintain a continuous pathway through a device.
Cross
An x-shaped union used to connect four pieces of tubing.
Cyano Phase
A specialized STATIONARY PHASE that consists of “cyano” (cyanopropylsilyl) groups bonded to the base silica.
Dead Volume
That portion of the flow path that is not directly Inline, that is unswept. These small spaces within the LC system allow remixing of the separated sample bands, as well as dilution of the initial sample with mobile phase. Dead volume should be minimized in a LC system, especially when small volume columns of high plate number are used.
Degassing
The process of removing dissolved gases from the MOBILE PHASE, to provide stable detection results. Often achieved through HELIUM SPARGING, vacuum filtration, inline vacuum degassing, and heating.
Delrin® (Acetal Resin)
A chemically-resistant polymer used to make fittings. This polymer cannot withstand high or low pH ranges. Delrin is a Registered Trademark of the DuPont Co. Please see our Polymer Information Chart for more information regarding this material.
Detector
One of the seven basic components of an HPLC system; responsible for providing quantitative data regarding the presence of the separated sample components as they pass through its flow cell.
Differential Refractive Index (RI) Detectors
Specialized detectors that identify changes in an ELUENT’s refractive index as it passes through the flow cell.
Drain Valve
Otherwise known as a purge valve, it is used to direct the pump fluid stream to waste for flushing or solvent changeover.
Effluent
The liquid MOBILE PHASE that leaves the column.
Electrochemical Detector
A specialized detector which is capable of monitoring an oxidation or reduction reaction with the confines of its flow “cell.”
Electro-Polishing
An Ultra High Purity process where 300 series Stainless Steel is electrochemically polished to remove all traces of surface contamination while smoothing the surface to low micro-inch levels. The process also develops a super passive Chromium Oxide layer that does not react with most liquids and gases.
Eluate
The combination of the MOBILE PHASE and the SOLUTE exiting the column.
Eluent
Another term for the MOBILE PHASE used to carry out a separation
Elution
A term used to describe the passing of MOBILE PHASE or ELUENT through a column tube for the purpose of moving SOLUTES through the system’s flow path.
Enantiomeric Compounds
Refers to a pair of compounds which exist as optical ISOMERS, or non-superimposable mirror images of each other.
End-capping
Refers to the procedure employed following the primary bonding process of the stationary phase onto the base packing material in a column. The purpose of this procedure is to remove residual silanol groups from the packing surface. This process typically helps increase a column’s overall CARBON LOAD.
End Fitting
Used with most standard columns in the market today, it is the fitting at the end of the column that allows commonly used tubing to interface with the column tube. Additionally, the end fitting often holds the frit in place on either end of the column tube, thus retaining the packing material within the column.
Extra-Column Effects
Those portions of the flow path outside the column itself that contribute to band broadening. Common culprit areas include the injection valve, the detector, the tubing and fittings used along the flow path, frits, etc.
External Standards
A special sample which contains known quantities of the same compounds of interest. Typically used to help identify sample peaks by comparing the time in which they eluted, to times obtained through the injection of the external standard under the same conditions.
Ferrule
A tapered conical ring used to make the seal between a piece of tubing and a receiving port. Ferrules almost invariably must be used in conjunction with a nut of some sort.
Fingertight
A special fitting invented by IDEX Health & Science that can be tightened to normal working HPLC pressures without the use of a wrench.
Fittings
Refers to the connectors that join tubing, columns, and various LC modules together.
Flanged Fitting
A fitting designed to be used in low to moderate pressure applications. The fitting requires flanging – or spreading the end of the tubing – before use. Often used with 1/4-28 or M6 threaded flat-bottom fittings.
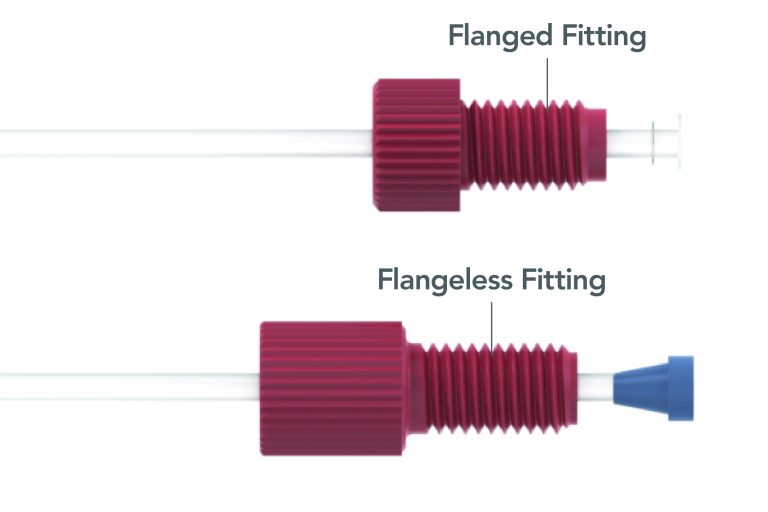
Flangeless Fittings
A specialized fitting designed to replace FLANGED FITTINGS, where through the use of a nut and unique ferrule, a seal can be made on tubing in areas where flanged fittings were traditionally necessary.

Flow Rate
Describes quantitatively the movement of fluid along a flow path, most often expressed in mL/min (milliliters per minute) for typical liquid chromatography applications.
Fluorescence Detector
Refers to a type of detection instrument often used in HPLC, in which sample components are exposed to a specific wavelength of light (often in the UV range). This exposure causes the sample components to “fluoresce,” or emit light of a different wavelength. This emitted light is detected by the fluorescence detector.
Frit
A porous metal or polymeric filtration component that is used to perform the following: a) prevent particulates from entering the LC system through the mobile phase, b) contain the column packing inside the column, or c) protect other parts of the LC system. Frits typically vary in their porosity from 0.5 micron (often used for inline filters protecting the column) to 20 micron (often used in high flow applications).
Fronting
Describes a non-GAUSSIAN curve in which the peak “leads in,” evidenced by slight tapering on the front part of the chromatogram’s trace. Fronting is the opposite of TAILING.
Gaussian Curve
Forms the basis for most current chromatographic theory. This term references the “perfect” curve, characterized by its symmetrical bell shape. Due to its very nature, however, a true Gaussian curve is rarely actualized in day-to-day chromatography.
Gradient Elution
An HPLC procedure where the composition of the mobile phase changes during the separation; opposite of ISOCRATIC ELUTION where the mobile phase composition remains constant throughout the entire analysis. A typical example would be where the mobile phase starts out as water, and methanol is added continuously so that the percentage of methanol in the mobile phase increases from zero to 100% during the run.
Guard Column
 A short column placed between the sample injector and the inlet of the main (“analytical”) column. The guard column is typically packed with the same kind of STATIONARY PHASE as the main column, and is intended to absorb
or pick up impurities (CHEMICAL GARBAGE) in the sample or mobile phase that might damage the main column. It is also sometimes called a PRE-COLUMN.
A short column placed between the sample injector and the inlet of the main (“analytical”) column. The guard column is typically packed with the same kind of STATIONARY PHASE as the main column, and is intended to absorb
or pick up impurities (CHEMICAL GARBAGE) in the sample or mobile phase that might damage the main column. It is also sometimes called a PRE-COLUMN.
Why Use Guard Cartridges
Guard columns protect your analytical column investment against deterioration from strongly or irreversibly adsorbed compounds or fouling by particulate matter. Symptoms of columns damaged
by strong or irreversible adsorption include changes in retention time and selectivity, offset baseline (bleed) and peak shape deterioration. Columns damaged by particulates (from the sample matrix, mobile phase, etc.) commonly exhibit
peak splitting and increased back pressure.
Guard columns protect your analytical column in two ways. First, they act as filters trapping particles in their frits or packed bed, or both. Second, when the guard column is packed with the same material as in the analytical column, it removes compounds that irreversibly or strongly bind to the packing material. By either approach, guard columns can increase your column life considerably.
Helium Sparging
The process of bubbling helium (a gas that does not dissolve noticeably into most solutions) through the mobile phase to remove gases which are dissolved into solution.
High Pressure Mixing
A pumping procedure in which two or more different solvents are mixed on the high pressure side of the pump to form a final mobile phase. Generally one pump is required for each solvent.
HiFloFrits
Most frits for HPLC use are made by sintering (heating but not melting) fine particles of stainless steel. The pores in the structure are the spaces between the particles and they allow liquid to pass freely. Frits exist for two reasons: to keep the packing material inside the tube and to keep particles in the sample or mobile phase from entering the column bed.
Hydrophilic
“Water-loving”; Refers to compounds, solvents or bonded phases that either dissolve easily in water or prefer water to non-polar organic solvents. See HYDROPHOBIC.
Hydrophobic
“Water-hating”; Refers to compounds, solvents or bonded phases that either dissolve easily in non-polar organic solvents such as hexane, or prefer such solvents to water. See HYDROPHILIC.
Injection Solvent
The solvent that the sample is dissolved into before it is injected; also known as the sample matrix.
Injector
One of the seven basic components of an HPLC system; allows a predetermined amount of sample to be introduced into the MOBILE PHASE flow path. The injector can be as simple as a manually-actuated valve for single injections, or it could be a completely automated instrument for unattended, multi-injection analyses.
Inlet Check Valve
The check valve(s) on an LC pump that allow(s) MOBILE PHASE to flow from the reservoir into the pump, but not in the reverse direction. See OUTLET CHECK VALVE and CHECK VALVE.
Inlet Filters
Filtration devices attached to the inlet lines of the pump that remove contaminants and particulate matter from the MOBILE PHASE before the solvent reaches the pump. Because of their construction, they are often referred to as “sinkers”.
Inline Check Valve
A specialized check valve that is placed anywhere along a flow path where limiting the flow to one direction is desired.
Inline Filter
A small volume device which prevents particulate matter, stemming from the sealing surfaces within the system or from the sample itself, from contaminating the system in any way. Often used between the pump and the injector to keep particles
shredding off the pump seal surfaces from contaminating the injector valve.
Interference
An unwanted band that overlaps one of the desired bands in a chromatogram. Interferences reduce the reliability of the results.
Internal Standards
Used primarily for the purpose of calibration. Internal standards consist of a known concentration of a sample, different from the actual analyzed sample, which help minimize volumetric errors that may occur during sample handling or preparation.
Ion Exchange Chromatography (IEC)
An HPLC method in which water and similar solvents are used as the primary solvents to ELUTE ions, acids and bases, following selective retention on the anionic and cationic sites within the column.
Ion Pair Chromatography
An HPLC method in which ions are “paired” with special agents, thus neutralizing the ions and allowing them to be thus retained on a standard reversed-phase column.
Isobore
Isobore features a surface finish (Ra) of <10 µinch with typical values around 8 µinch – twice the smoothness of standard LC grade. This highly polished tubing is recommended for use with 3 and 5 µm packing materials (and smaller) and with small internal diameter columns (<3.0mm inner diameter). Iso-Bore™ Surface Finish
Isocratic Elution
Refers to an HPLC separation where the mobile phase composition stays the same during the entire analysis. Isocratic separations are the opposite of GRADIENT ELUTION separations.
Isomers / Isomeric Compounds
Distinctly different compounds with different properties which share the same molecular formula.
Linear Velocity
Related to the speed at which fluid — and thus sample components — moves along a flow path, typically reported as cm/min or mm/sec. The linear velocity calculation allows adaptation of methods when changing from one column diameter to another, as it is vitally important to keep the linear velocity the same as column diameters are changed.
Liquid-Solid Chromatography
An HPLC method, different from REVERSED-PHASE LC, in which mobile phases that do not include water are used. See also ADSORPTION CHROMATOGRAPHY.
LiteTouch®
An innovative, high pressure fitting with a reusable polymer ferrule. LiteTouch is a Registered Trademark of IDEX Health & Science.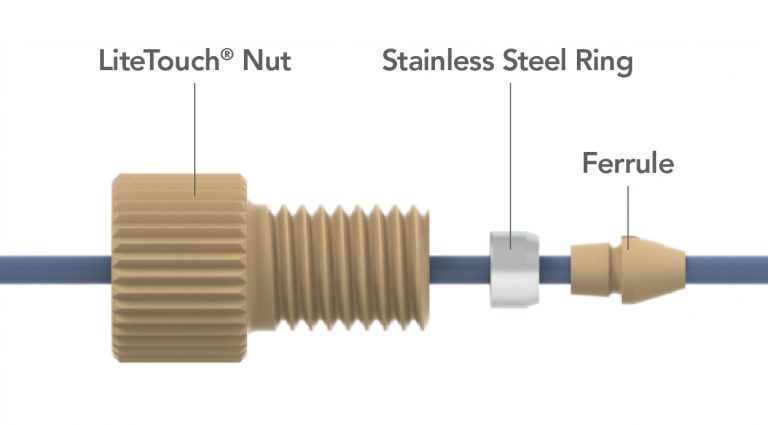
Loop
A piece of tubing connected to a sample injector that holds the volume of sample to be introduced into the flow path. When used in full-injection mode, the loop establishes the analyzed volume.
Low Pressure Mixing
A pumping procedure that mixes two or more solvents before the pump to form a MOBILE PHASE and then delivers the mixture to the LC system. Often just a single pump is used, or a single pump plus a controller.
Mass Spectrometry
A chromatographic analysis tool in which sample components are ionized and resolved on the basis of their mass to charge (m/z) ratio due to their behavior in electric and magnetic fields. At one time, instruments used in this technique were cost prohibitive, but new technology and advances in the technique are constantly reducing the cost (and size) of equipment used for this analysis. Currently, mass spectrometry is often coupled with other separation techniques, like gas and liquid chromatography systems, to serve as a detection source.
Mass Transfer
Refers to the movement of SOLUTE into and out of the STATIONARY PHASE or MOBILE PHASE; the faster the mass transfer value, the better the efficiency of the column. Mass transfer can be affected by numerous factors, including stationary phase particle size, as well as MOBILE PHASE temperature and viscosity.
MegaPascal (MPa)
A unit of pressure; one MPa equals about 10 bar (atmospheres) or 150 pounds per square inch (psi). Please see our Pressure Conversion Table.
Microbore
Term typically applying to columns used in HPLC applications where the inner diameter is less than or equal to 2 mm. It also is used describing a family of HPLC applications in which this size of column is used.
Mobile Phase
One of the two general “phases” described in an HPLC system, this refers to the solvent that is moving (or is “mobile”) through the system. See also STATIONARY PHASE.
Mobile Phase Strength
Determines how fast the sample moves through the column; a STRONG MOBILE PHASE results in sample bands coming out fast, and a WEAK MOBILE PHASE gives longer retention times for each band.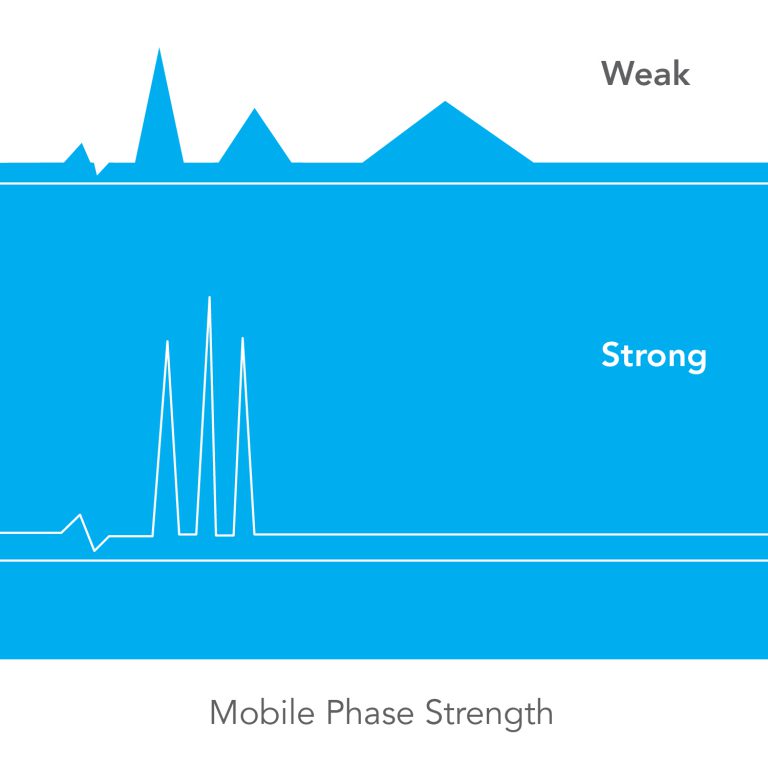
Modular
This is the term used for the IDEX Health & Science threaded column hardware. Modular HPLC hardware is designed to be even more user-friendly than ordinary compression style hardware. With modular hardware, there is no need to apply precise torque to get the ferrule to grip the tube. The high pressure seal is instead formed by the threads on the tube and end fitting. There is also no problem of the frit not seating in the end fitting. The frit is contained in a cap that slides over the column forming a leak and gap-free seal. IDEX Health & Science modular hardware also eliminates safety concerns when packing at high pressures.
Modular Bio-Safe
Bio-Safe is a IDEX Health & Science trade name. This is also called a peek modular system. IDEX Health & Science Bio-Safe™ modular columns are biocompatible and precision machined from virgin PEEK (polyetheretherketone), a strong, inert polymer material.
Noise
In HPLC, this usually refers to an irregular BASELINE that is hard to measure. All baselines become “noisy” if the attenuation of the detector is decreased enough.
Non-Polar
Compounds, solvents or bonded phases that dissolve readily in solvents, such as hexane, or prefer such solvents in place of water. Non-polar substances do not dissolve in water.
Normal Phase Chromatography
An HPLC method in which a non-polar mobile phase is used with a polar stationary phase; opposite of REVERSED-PHASE CHROMATOGRAPHY.
Outlet Check Valve
The check valve in an LC pump that allows mobile phase to flow to the column from the pump, but not in the reverse direction. See CHECK VALVE.
Packing Material
Generally refers to the STATIONARY PHASE, but more specifically to the adsorbent, gel or solid support used in the column itself.
Partially-Resolved Peaks
A term describing a phenomenon where multiple PEAKS run together and do not begin and end on the BASELINE. In order for a peak to be considered completely resolved, it must begin and end on the baseline without interference from other PEAKS.
Particulate
Refers to small, solid particles in the sample or mobile phase which can plug or damage the column or other parts of the HPLC system.
Peak
The visual representation on the chromatogram based on the detector’s electrical response due to the presence of a sample component inside the flow cell.
PEEK (Polyetheretherketone)
A chemically-resistant polymer used to make fittings and tubing; serves as an excellent replacement for stainless steel in biocompatible applications. Please see our Polymer Information page for more information regarding this material.
Why PEEK?
PEEK columns are used when analytes are sensitive to the metals in conventional stainless steel hardware. Proteins, peptides and ions have this susceptibility. Interaction of sensitive biomolecules with
metal can lead to loss of biological activity, loss of sample from adsorption, low efficiency and poor peak shape. PEEK is also compatible with most mobile phases used in reversed phase HPLC, with the exception of THF. Bio-Safe PEEK
tubing also has superior wall thickness for strength, and an ultra-smooth internal surface finish for high efficiency.
Like the modular stainless steel line, Bio-Safe columns are threaded and provide a safe column format for high pressure packing up to 8000 psi. IT also offers a packing adapter for the Bio-Safe line.
PEEK Capillary Tubing
Extruded from PEEK (polyetheretherketone) to precise tolerances, Peek capillary tubing is inert to most organic solvents however; DMSO, methlyene chloride and THF will cause PEEK tubing to swell and should be avoided with smaller internal diameters to prevent backpressure increase.
PK (polyketone)
A proprietary polymer blend comprised mainly of polyetheretherketone (PEEK). PK demonstrates all of the superior chemical resistance of PEEK (see PEEK above). The proprietary blend will however, allow a fitting to attain a higher pressures while reducing the cold flow properties of pure PEEK. CAUTION: some fittings molded of PK are known to be conductive. Use caution when employing PK fittings in high voltage applications. Please see our Polymer Information page for more information regarding this material.
Photodiode Array Detectors
Specialized, high-end UV/Vis detectors which record the absorbance of light at many wavelengths simultaneously.
Plate Number (N)
A value that describes how good a column is in keeping sample bands narrow. Columns with large plate numbers give narrow bands; long columns packed with small particles give the highest plate numbers.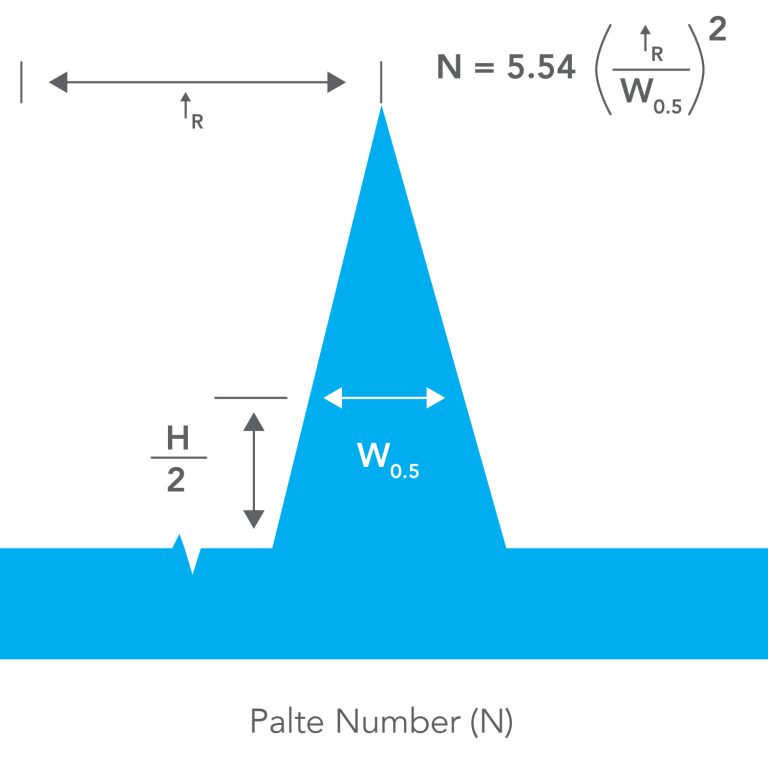
Polar
Compounds, solvents or bonded phases that either dissolve in water or prefer water to non-polar organic solvents such as hexane.
Polypropylene
A soft polymer used to make low pressure fittings. Please see our Polymer Information page for more information regarding this material.
Pore
A term which refers to small passageways or tunnels that criss-cross or “honeycomb” a particle. The term is typically used in reference to the small particles which make up the stationary phase of a column. Additionally, the term may also refer to the passageways in a frit or filtering surface.
PPS (polyphenylene sulfide)
A hard polymer, relatively new to the analytical lab equipment market, used to make fittings. Please see our Polymer Information page for more information regarding this material.
Precolumn
A special, small column — typically packed with silica — which is connected between the pump and the sample injector. It is designed to protect the analytical column’s packing from aggressive high or low-pH mobile phases by saturating the mobile phase with silica. This minimizes further dissolution of the column packing and helps preserve the analytical column.
Precolumn Filter
A filter used between the injector and the column (or guard column) to keep unwanted sample components from reaching the column.
Preparative Column
A larger-diameter column used for recovering purified materials by chromatographic separation; can be contrasted with “analytical columns” which are used mainly for analysis, rather than recovery of fractions.
PCTFE
A chemically-resistant fluoropolymer used to make fittings and accessories for fluid transfer applications. It is molecularly related to PTFE. Please see our Polymer Information page for more information regarding this material.
Pump Preload
A device used just after some pumps to maintain enough backpressure for reliable check valve operation.
Qualitative Analysis
The determination of which compounds are present in a sample. In LC, this is usually done by comparing retention times for bands in the unknown sample with retention times for various pure compounds suspected to be present in the sample.
Quantitative Analysis
The analysis of a sample to determine the concentrations of different compounds in the sample. Quantitative analysis in LC is usually based on the size of the bands in the sample chromatogram (peak height or area) compared to band size of a sample with a known concentration (the calibrator or standard).
Quaternary-Solvent Mobile Phase
A MOBILE PHASE composed of four solvents.
Ra
Surface smoothness or finish is often measured in Ra, or roughness average. Ra is the average difference between peaks and valleys on the surface. It can be measured electronically or optically. The smoother the surface, the smaller is the difference between its peaks and valleys and the smaller its Ra value. Ra is often reported in micro-inches (µinch).
Recovery
A term describing the amount of a sample that actually elutes from a column relative to the amount originally introduced into the system, typically described as a “percent.” This is particularly important with some biological analyses, where certain sample components tend to “hang up” in the stationary phase and bind active sites for future analyses.
Refractive Index (RI) Detector
A less popular detector based on change in refractive index of mobile phase due to the presence of sample. RI is less sensitive than photometric detectors for most compounds, and cannot be used with gradient elution. However, it is closest to a “universal” detector, in that most compounds will give a response at sufficiently high concentrations. Used mainly for size-exclusion chromatography.
Regeneration
A process in which the packing inside the column is restored to its beginning state following a gradient elution. It can also refer to the process of bringing back any column to its original state, usually following temporary damage to the bonded phase.
Residual Silanols
Refers to the silanol (SiOH) groups remaining on the surface of the silica beads following the process in which a phase is chemically bonded onto the silica bead surface. Residual silanols can interfere with reversed-phase analyses, as they provide slightly polar sites on the surface of the silica beads which can compete with the polar mobile phase during sample component separation. Residual silanols are often minimized through a process of END-CAPPING.
Resolution (RS)
Defines how well separated two adjacent bands are. Larger values of resolution mean better separation.
Retention Time (tR)
The time between sample injection (time zero) and the appearance of the band maximum; when all conditions are held constant, the retention time for a given band (or compound) remains constant.
Retention Volume (VR)
Simply stated, it is the volume of MOBILE PHASE required to elute a substance from the column.
Reversed-Phase Chromatography
The most common form of HPLC, in which water-based/polar MOBILE PHASES are used with special column packings that have a HYDROPHOBIC surface layer.
Sample Capacity
This refers to the amount of sample that can be introduced into an LC column before overload occurs. Column overload is defined as a condition where the efficiency of that column goes below 90 percent of its normal value.
Sampling Rate
A term describing how frequently the detector generates a reading through checking the flow cell. The proper sampling rate is often a very sensitive setting, as too fast a rate can generate more data than necessary and too slow a rate may allow a narrow band to be missed.
SealTight™
A dual-compression, high pressure fittings system manufactured by IDEX Health & Science. SealTight is a Trademark of IDEX Health & Science, LLC.
SealTight™ ferrule:
Semi-Preparative Chromatography
Refers to small-scale preparative chromatography typically utilizing a standard analytical or slightly larger column.
Sensitivity
Generally refers to detector sensitivity, which is the ability of the detector to give larger bands (other factors equal) and a better signal-to-noise ratio. This provides more precise analyses of very small concentrations of sample.
Separation Factor
Defined as the relative retention measured for two adjacent peaks, either through measurement of the difference between the centers of the peaks or between the tops of the peaks. Also referred to as selectivity.
Silanol
Refers to a SiOH group on the surface of the silica used in most columns.
Sinker
The inlet filter that connects to the end of the line that feeds MOBILE PHASE to the LC pump. Also serves to anchor the inlet line to the bottom of the reservoir.
Size-Exclusion Chromatography (SEC)
An HPLC method, different from reversed-phase chromatography, used mainly to separate high-molecular-weight samples and to determine their molecular-weight distribution. Also called Gel Filtration Chromatography (GFC) or Gel Permeation Chromatography (GPC).
Slurry Packing
Refers to the most common technique to pack an HPLC column; involves the use of a slurry (a suspension of the packing material and solvent).
Solute
Refers to a sample component that is separated using a column.
Solvents
The pure components of the MOBILE PHASE (e.g., water, methanol, acetonitrile, etc.). In reversed-phase chromatography, water is a WEAK SOLVENT, so MOBILE PHASES with higher concentrations of water are weaker and give longer retention times for all sample bands. Methanol is a STRONG SOLVENT; increasing the amount of methanol in the MOBILE PHASE makes it stronger, and sample bands leave the column sooner.
Solvent Strength
Refers to the ability of a solvent to elute a particular sample component or SOLUTE from a column.
Stainless Steel
Any variety of steel alloys designed for corrosion resistance. The different varieties – primarily 316 grade – are used to manufacture high-pressure, chemically-resistant HPLC fittings and tubing.
Standard
A sample with a known concentration of some compound X that is to be determined by an LC procedure. The concentration of X in a sample is determined by comparing its peak size with the peak size of the standard, usually by comparing the area underneath the peak.
Stationary Phase
The particulate material packed inside the column. Also, the bonded phase that is part of the column packing.
Stocking Distributors
A distributor that carries inventory of select catalog parts for faster delivery. Contact distributor for availability and pricing.
Strong Solvent/Mobile Phase
A strong MOBILE PHASE is one that give shorter retention times for all sample bands; in reversed-phase chromatography, methanol and acetonitrile are strong solvents, and water is a weak solvent. MOBILE PHASES with more methanol or acetonitrile
will be stronger than mobile phases with more water. So, 80 percent methanol/water is a stronger mobile phase than 20 percent methanol/water.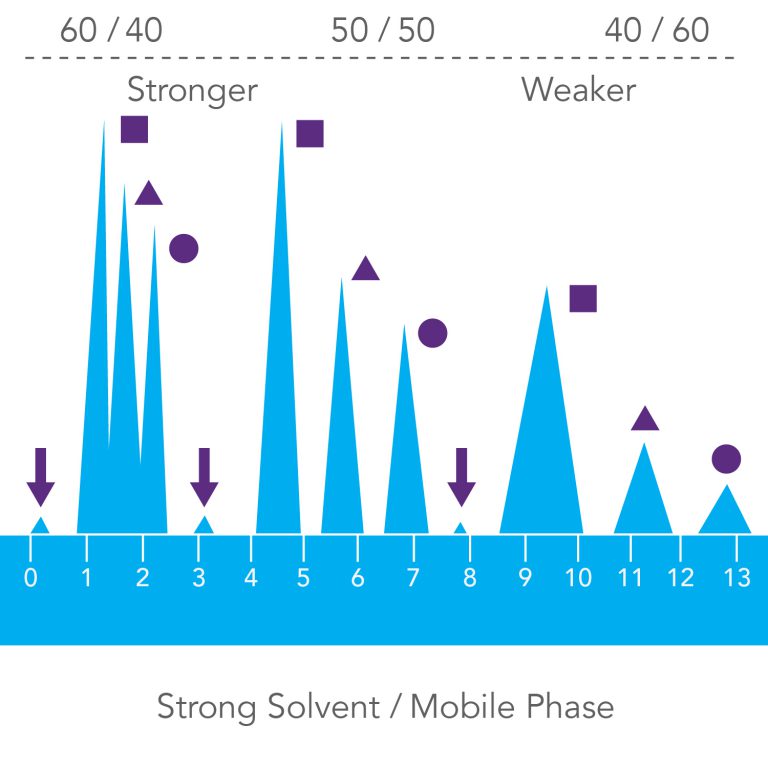
Supercritical Fluid Chromatography (SFC)
An HPLC technique which employs the use of supercritical fluids as the mobile phase to enhance the separation of samples that are typically problematic for standard LC analysis.
Supercritical Fluid Extraction (SFE)
A special type of mixture extraction employing the unique benefits of supercritical fluid. Related to SUPERCRITICAL FLUID CHROMATOGRAPHY (SFC).
Swaging
This is the term used for tightening a compression fitting onto a compression tube.
Swept Volume
The spaces along a flow path that contain no dead volume, and thus allow no mixing of the sample.
Tailing
A term used to describe the asymmetrical shape of a peak, technically defined as having an asymmetry factor >1.
Tee
A T-shaped fitting used to connect three pieces of tubing.
Titanium
Titanium products were created initially as a biocompatible alternative to stainless steel — something that did not have ferrous components. While being a metal and therefore offering a higher temperature rating than polymers can offer, titanium does not exhibit the same strong chemical resistance that stainless steel does, and is particularly susceptible to attack by strong acidic or basic solutions. There are many other solvents that will interact with titanium, so it is recommended that a chemical compatibility chart be consulted.
Void (or Column Void)
A space in the column packing, generally at the inlet of the column, or also a settling of the packing at the column inlet to create a space between the top of the packing and the frit. A void usually makes the column unsuitable for use.
Void Volume
The total internal volume of a connection or fluid pathway. DEAD VOLUME + SWEPT VOLUME = VOID VOLUME.
Waste Container
Listed as an essential component for any HPLC system, it is a vessel of sorts used at the end of the system used to collect mobile phase and separated sample components.
Weak Solvent/Mobile Phase
A weak MOBILE PHASE gives longer retention times for sample bands, compared to a strong MOBILE PHASE. Water is a weak solvent and methanol and acetonitrile are strong solvents for reversed-phase chromatography. MOBILE PHASES with more water will be weaker, and MOBILE PHASES with more organic solvent will be stronger. A 20 percent methanol/water mobile phase is weaker than a 80 percent methanol/water MOBILE PHASE. See also STRONG SOLVENT.
Zero Dead-Volume (ZDV)
In theory, a fitting which adds no extra-column volume to the system when making a connection. Commercially-manufactured ZDV fittings actually have a finite, but insignificant, dead volume in terms of practical chromatography.